Multi-Platform, Multi-Method DNA Library Preparation
Since DNA was identified as the genetic material of life, scientists have pursued its study with ever-growing enthusiasm. The discovery of the DNA double-helix structure by Watson and Crick in 1953 marked a groundbreaking leap in our understanding of life, catalyzing rapid advancements in DNA sequencing technologies.
• Key Milestones in DNA Sequencing Technology
From the first-generation Sanger method (1977) to Roche's pyrosequencing-based second-generation technology (2005), followed by Illumina's NovaSeq™ series (2017) and the MGISEQ-T7 ultra-high-throughput system (2018), the field has witnessed remarkable progress. The emergence of third-generation platforms like PacBio and Oxford Nanopore enabled real-time, single-molecule long-read sequencing, overcoming previous fragment length limitations. DNA sequencing technology has evolved dramatically over these four decades, now empowering transformative applications across genomics, medicine, and beyond.
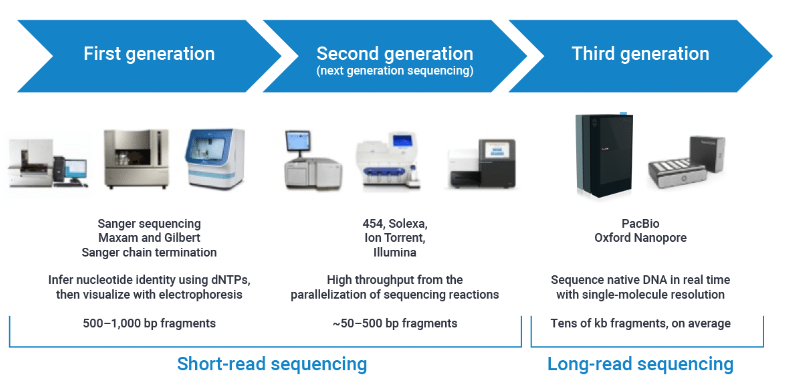
Image source: online
• Second-Generation Sequencing Platforms
Second-generation sequencing, also known as high-throughput sequencing (NGS), is based on the core principle of "sequencing by synthesis." Its key features include the ability to simultaneously analyze hundreds of thousands to millions of DNA strands, enabling efficient and cost-effective transcriptomic and deep genomic sequencing while maintaining high accuracy. However, this technology still relies on PCR amplification, which introduces potential biases, and suffers from shorter read lengths. Major commercial platforms include Illumina's HiSeq series, Thermo Fisher's Ion Torrent, and BGI's MGISEQ series, with Illumina dominating the global market at an 83.9% share.
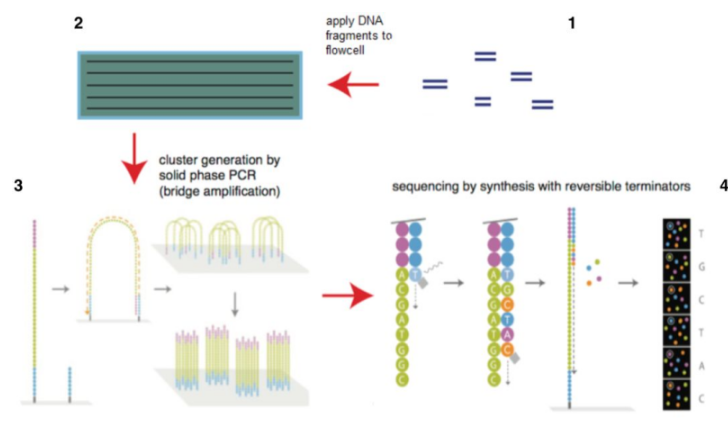
Illumina Sequencing Technology (Source: Illumina Official Website)
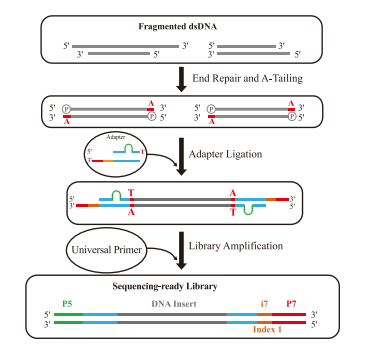
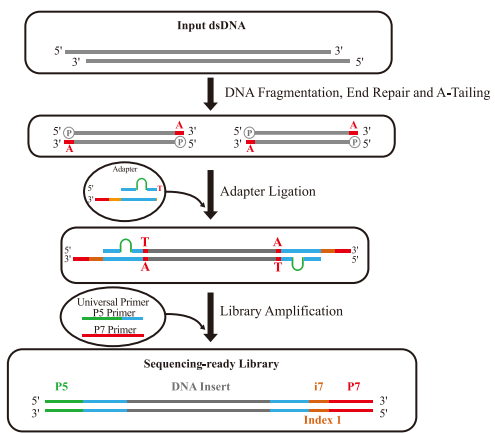
With the rapid advancement of high-throughput sequencing technologies, demands for both sequencing quality and throughput have significantly increased. The NGS workflow primarily consists of three key steps: library preparation, sequencing, and data output. Among these, DNA library construction serves as the foundation of NGS. Efficient and streamlined library preparation methods are critical for optimizing overall sequencing efficiency. The core principle involves fragmenting target DNA samples into smaller segments and ligating adapters to their ends. Depending on the fragmentation method and adapter ligation strategy, common library preparation approaches include:
Conventional DNA library preparation
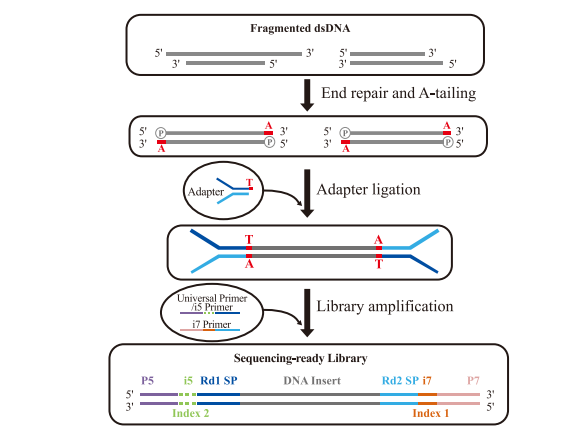
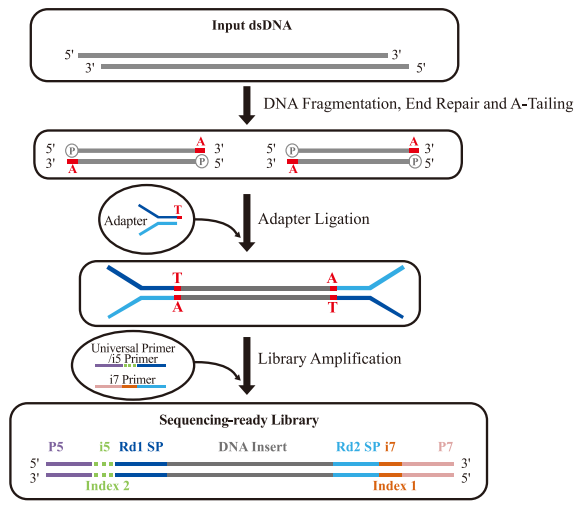
Conventional DNA library preparation is widely used in NGS due to its broad sample compatibility and high library conversion efficiency. This method primarily employs TA cloning-based adapter ligation, with the following workflow:
1.DNA Fragmentation
2. End Repair & A-Tailing
3. Adapter Ligation
4. Library Amplification
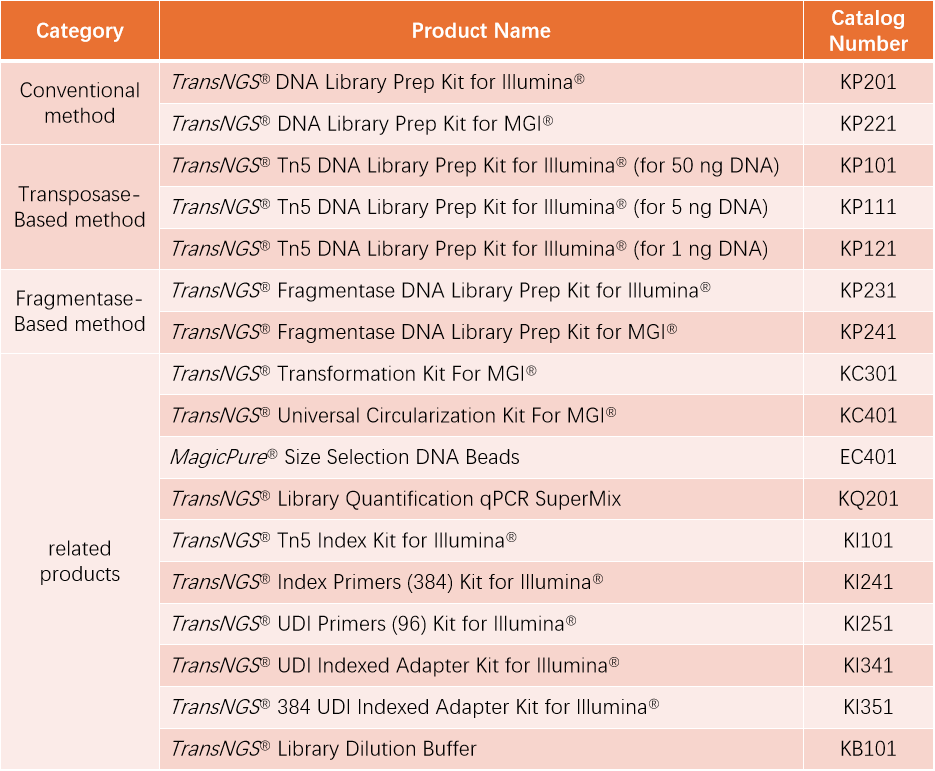
TransGen offers two optimized kits compatible with Illumina and MGI platforms, supporting diverse applications including: Whole-genome sequencing, Targeted gene panels, Metagenomics, etc.
TransNGS® DNA Library Prep Kit for Illumina®
This product is specifically developed for Illumina high-throughput sequencing platforms, designed to efficiently and rapidly convert 1 ng–1 μg of fragmented dsDNA into sequencing-ready libraries. It features high library conversion efficiency and broad sample compatibility.
Schematic Diagram of Library Construction Principle
i5 position with dotted line indicates some libraries do not have this index
Comparative Analysis with Competing Products
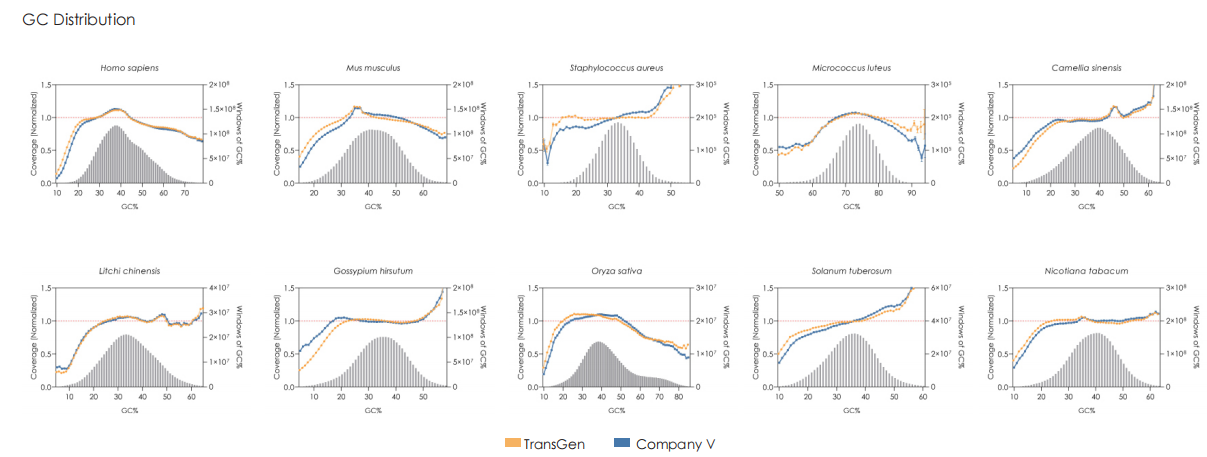
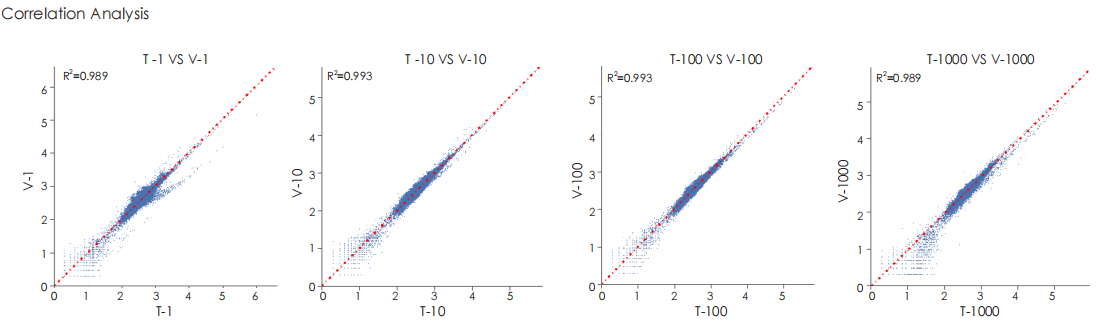
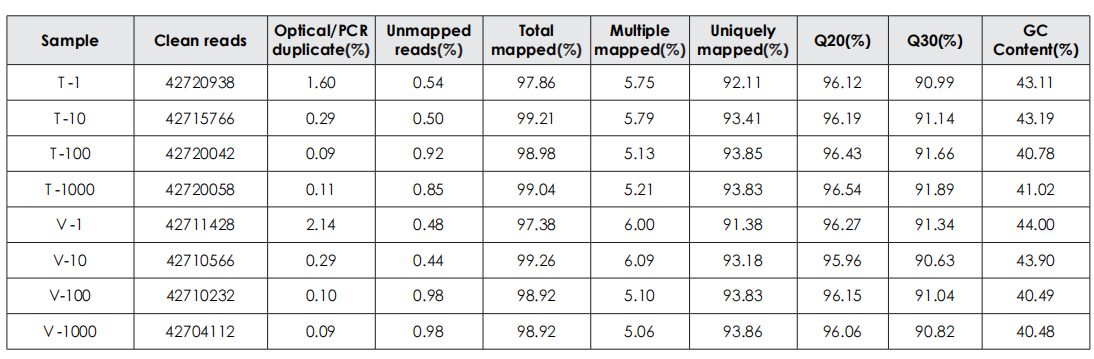
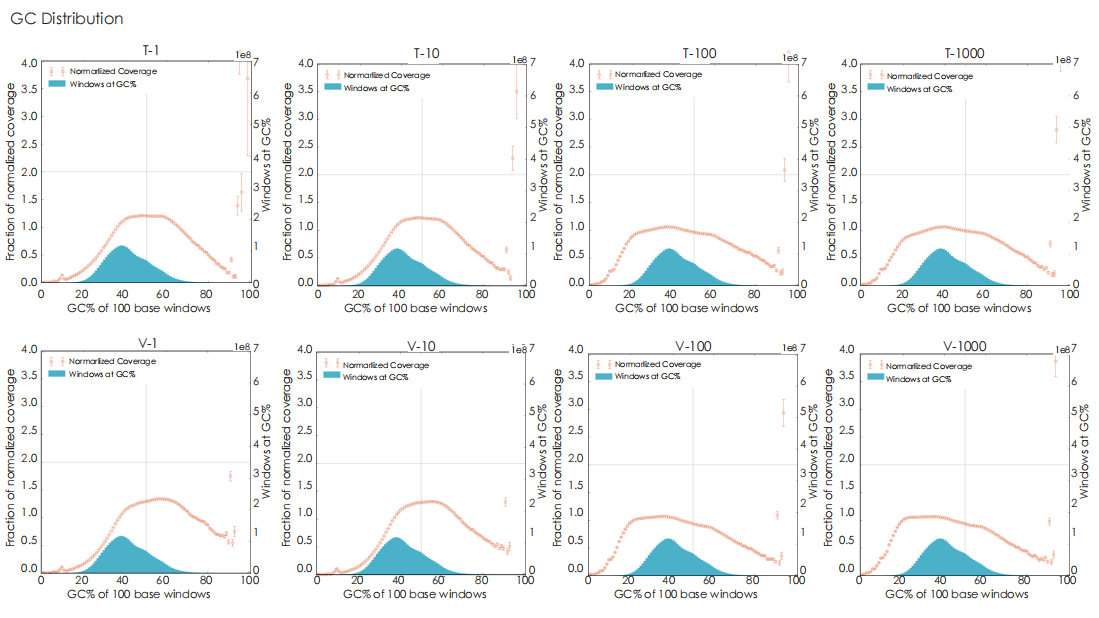
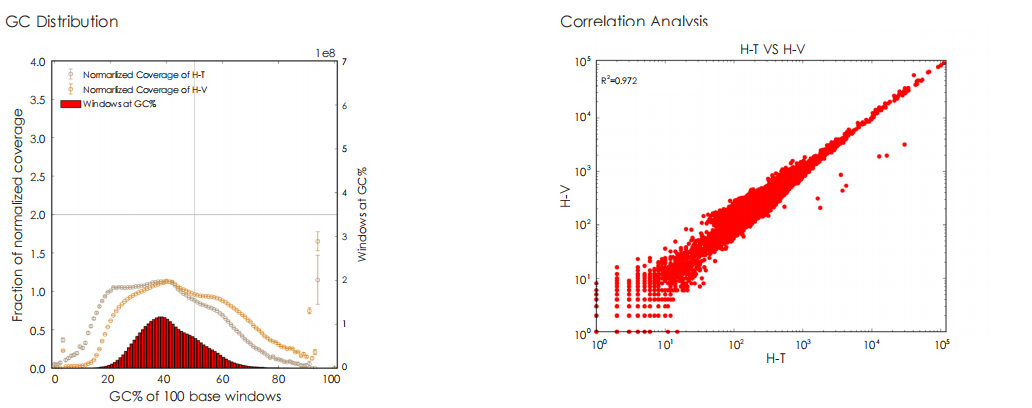
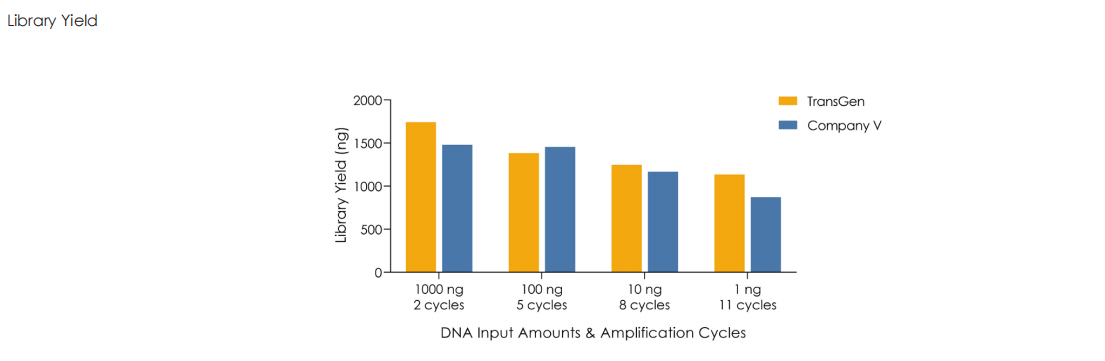
A head-to-head comparison was conducted between TransGen and Company V DNA library prep kits using sheared HeLa cell DNA (input: 1 ng, 10 ng, 100 ng, and 1000 ng). The TransGen kit demonstrated superior library yield while maintaining comparable library peak profiles. Subsequent sequencing analysis revealed no significant differences in key metrics including Q20/Q30 scores, GC content, duplicate rates, alignment rates, and GC distribution. Sequencing results showed high correlation (R² > 0.95) between the two kits, confirming equivalent data quality.
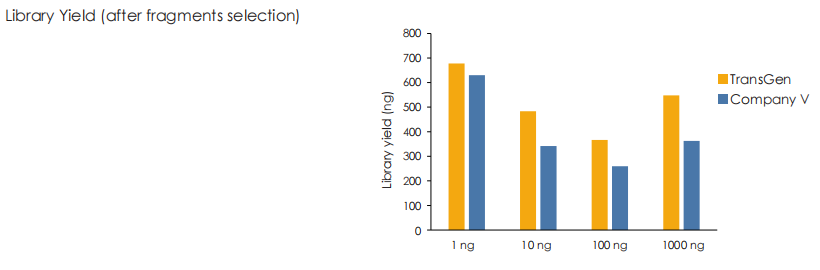
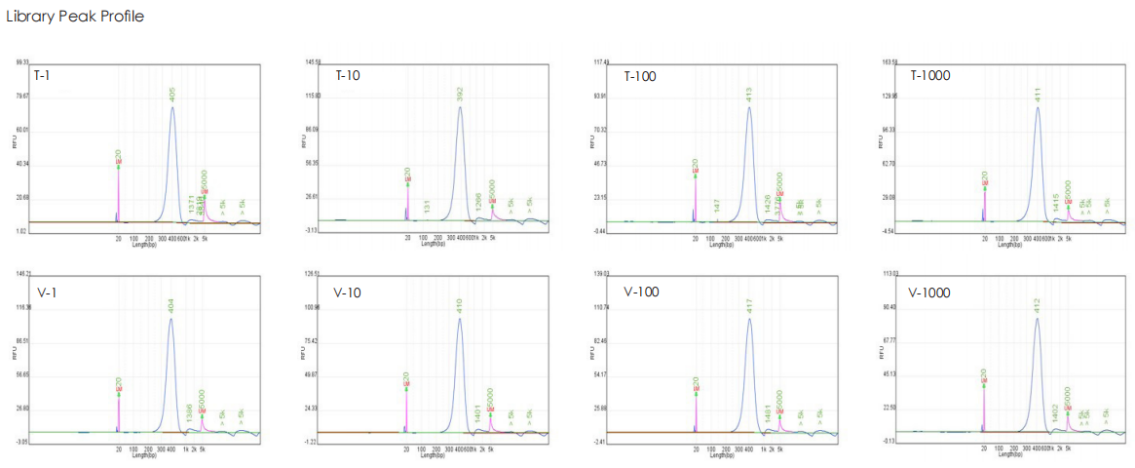
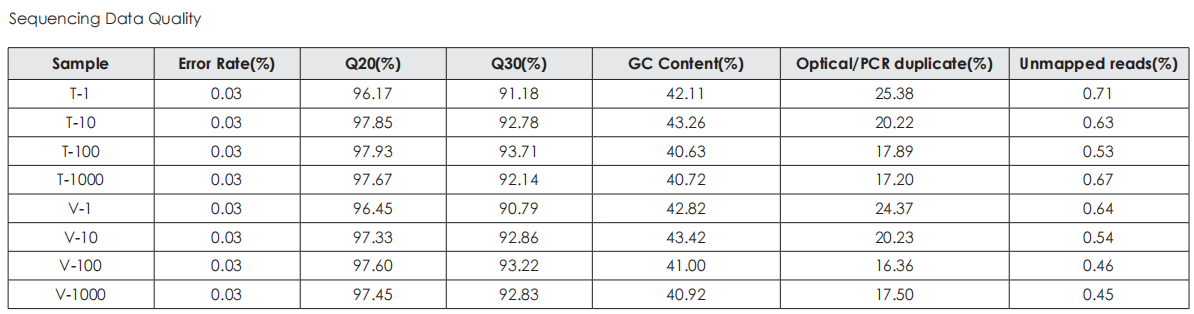
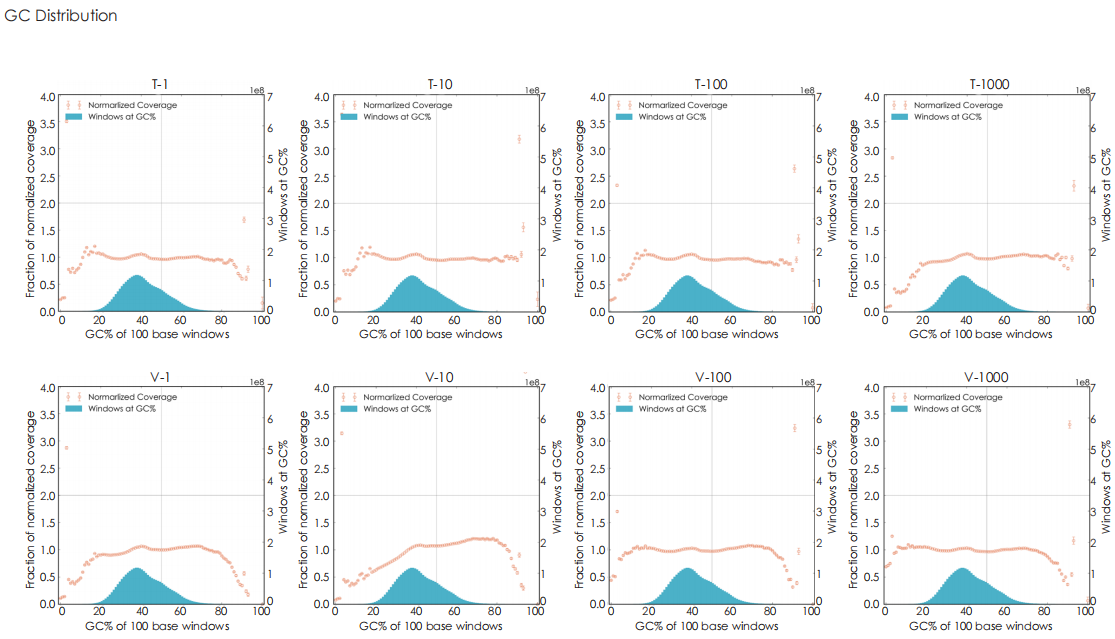
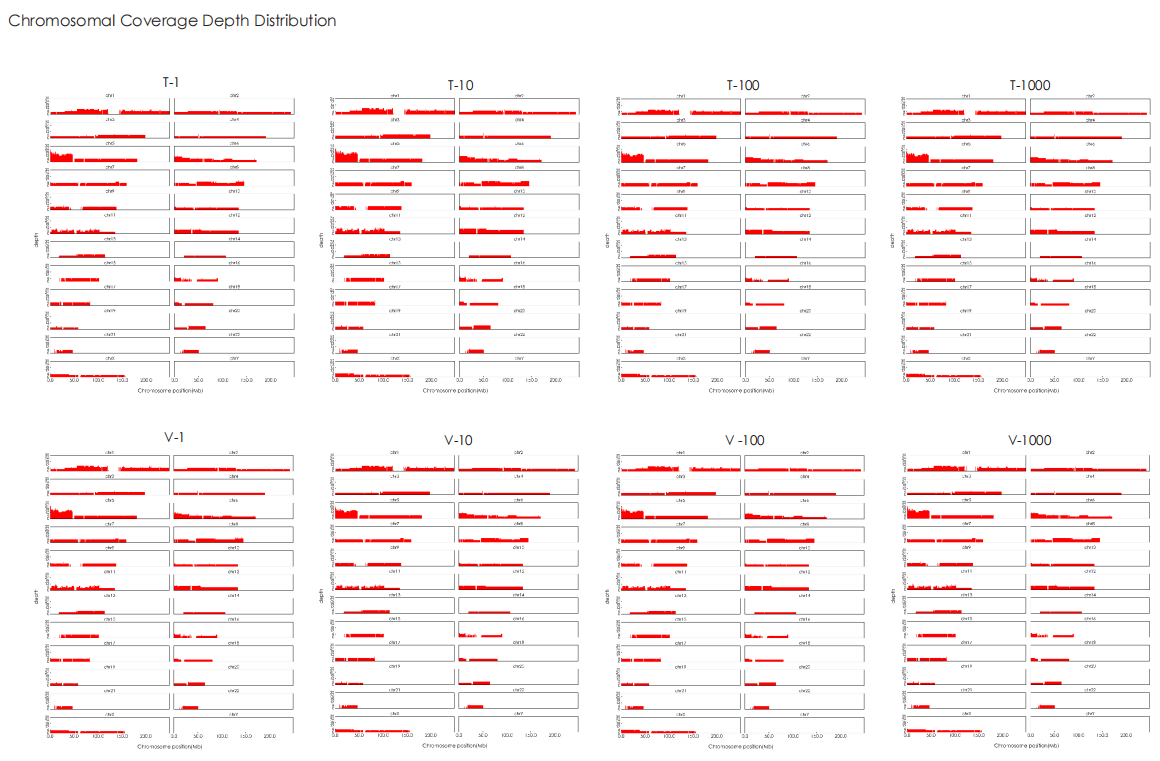
TransNGS® DNA Library Prep Kit for MGI®
This product is a high-performance library preparation kit specifically optimized for MGI high-throughput sequencing platforms. It delivers exceptional library conversion efficiency, broad sample compatibility, and high-quality sequencing data.
Schematic Diagram of Library Construction Principle
Comparative Analysis with Competing Products
(1) Library Yield with Different Input Amounts
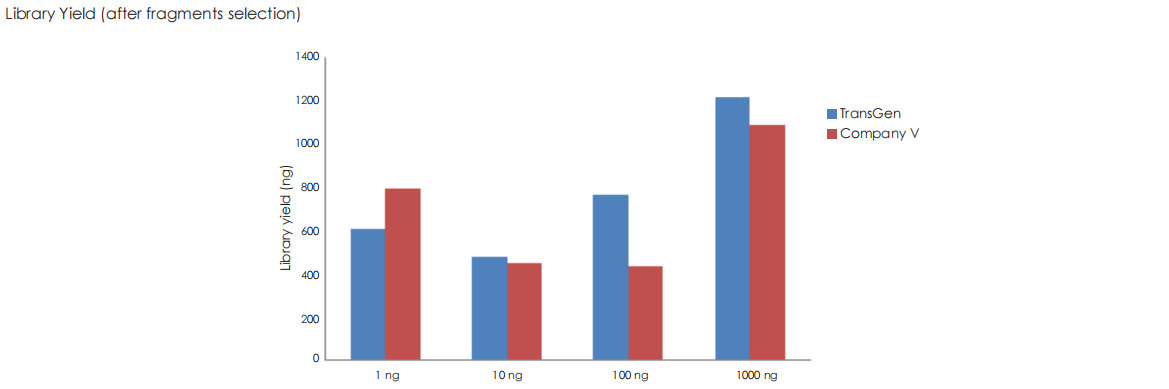
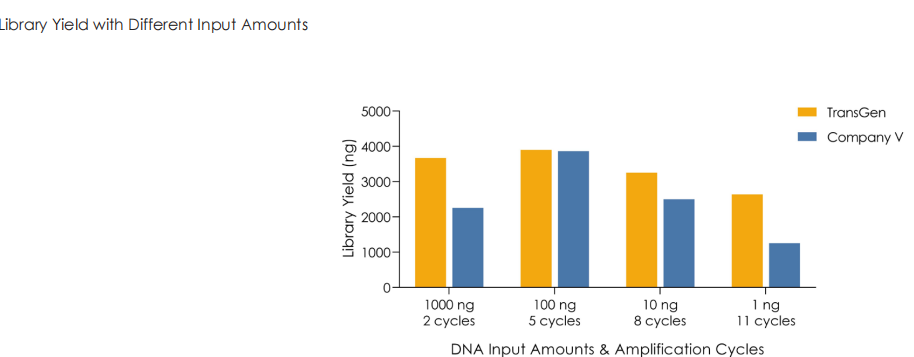
Using TransGen and Company V kits, fragmented dsDNA derived from human HeLa cells is subjected to library construction with identical PCR cycles across varying input amounts (1 ng to 1 μg). Results demonstrate that the TransGen kit achieve consistently high library preparation efficiency across the entire input range (1 ng–1 μg).
(2) Comparison of sequencing result
TransGen and Company V products were used for dsDNA library construction using different amount of fragmented Hela cell DNA. The results showed that the quality of sequencing result, GC distribution and correlation generated by TransGen is as good as competing products.
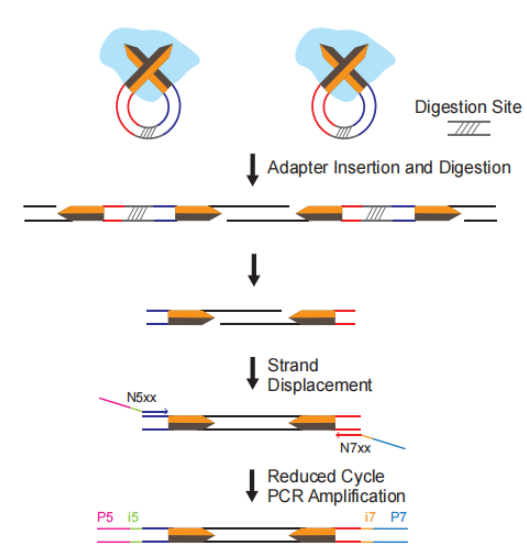
Transposase-Based Library Preparation
Unlike conventional library preparation—which requires separate DNA fragmentation, end repair, and adapter ligation steps—in vitro transposition technology offers a rapid, streamlined alternative. This method simultaneously fragments DNA and ligates adapters in just 5 minutes, significantly reducing hands-on time while maintaining compatibility with low-input samples (as little as 0.1 ng). To accommodate varying input amounts, TransGen offers three optimized Tn5-based library prep kits:
✼ TransNGS® Tn5 DNA Library Prep Kit for Illumina® (for 50 ng DNA)
✼ TransNGS® Tn5 DNA Library Prep Kit for Illumina® (for 5 ng DNA)
✼ TransNGS® Tn5 DNA Library Prep Kit for Illumina® (for 1 ng DNA)
Schematic Diagram of Library Construction Principle
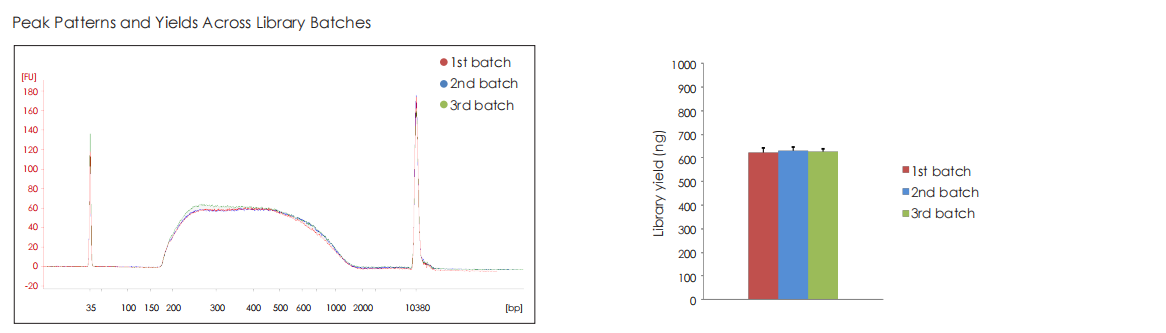
High Stability
Whole-genome libraries are constructed from 5 ng of human genomic DNA using kits of different batches. After 9 PCR cycles, the products are purified using 1.0× DNA magnetic beads (TransGen, EC401) (without size selection). The libraries are then analyzed using an Agilent High Sensitivity DNA chip, showing consistent fragment size distributions, primarily ranging between 200–2000 bp. The library concentrations are measured by Qubit, demonstrating consistent yields across batches. The uniformity in peak profiles and yields confirms the high batch-to batch consistency of the product.
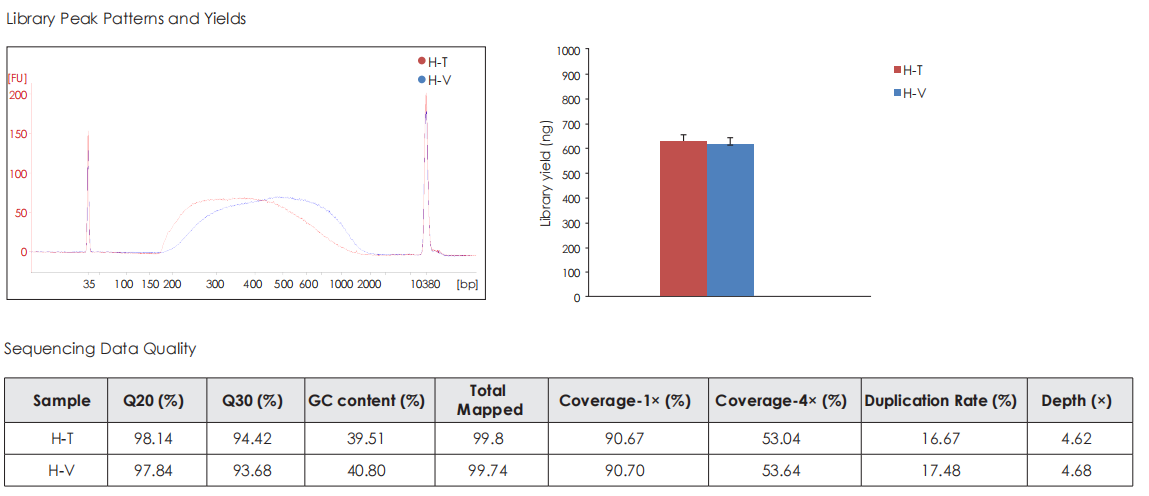
Comparative Analysis with Competing Products
Using 5 ng of human genomic DNA as input, whole-genome libraries are prepared with TransGen and Competitor V’s products, followed by library sequencing and analysis. The results demonstrate that TransGen’s product exhibits comparable performance to the competitor in terms of library peak profile, yield, sequencing data quality, GC distribution, and correlation.
Fragmentase-Based Library Preparation
Compared to ultrasonication, enzymatic fragmentation offers simpler operation (fewer tube transfers), lower sample loss, and enhanced compatibility with low-input samples (down to 1 ng). Critically, it eliminates the need for specialized instruments or consumables, significantly reducing costs. To leverage these benefits, TransGen has launched two new enzymatic fragmentation-based library prep kits, optimized for Illumina and MGI platforms:
TransNGS® Fragmentase DNA Library Prep Kit for Illumina®
This product enables rapid and efficient DNA library construction from 1 ng to 1 μg of input dsDNA, integrating DNA fragmentation, end repair, and A-tailing into a single-step reaction. The resulting product requires no intermediate purification and can be directly used for adapter ligation.
Schematic Diagram of Library Construction Principle
i5 position with dotted line indicates some libraries do not have this index
Comparative Analysis with Competing Products
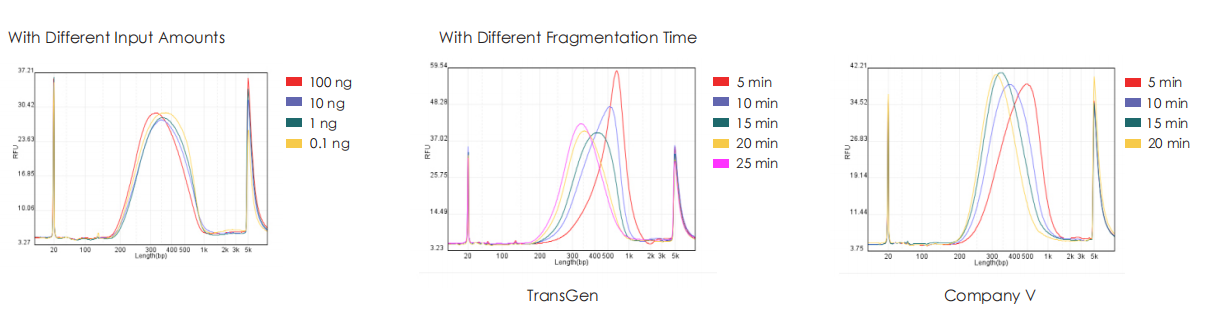
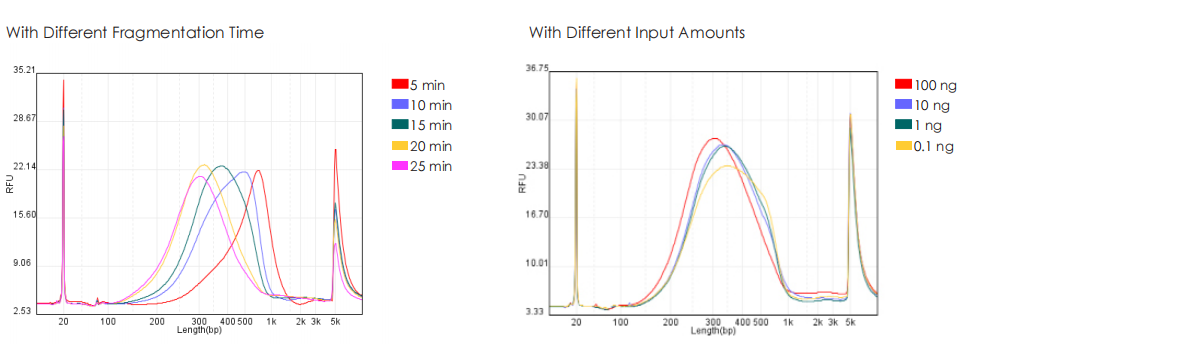
Genomic DNA from HeLa cells is used to construct libraries with TransGen and Company V kits separately. Results show that libraries generated by the TransGen kit exhibit consistent peak profiles across input amounts (0.1 ng, 1 ng, 10 ng, and 100 ng). When fragment size is modulated by adjusting fragmentation time (50 ng input), the TransGen kit demonstrates more sensitive peak profile shifts compared to Company V’s. Additionally, at inputs of 1 ng, 10 ng, 100 ng, and 1000 ng, the TransGen kit achieves comparable or superior library yields relative to Company V.
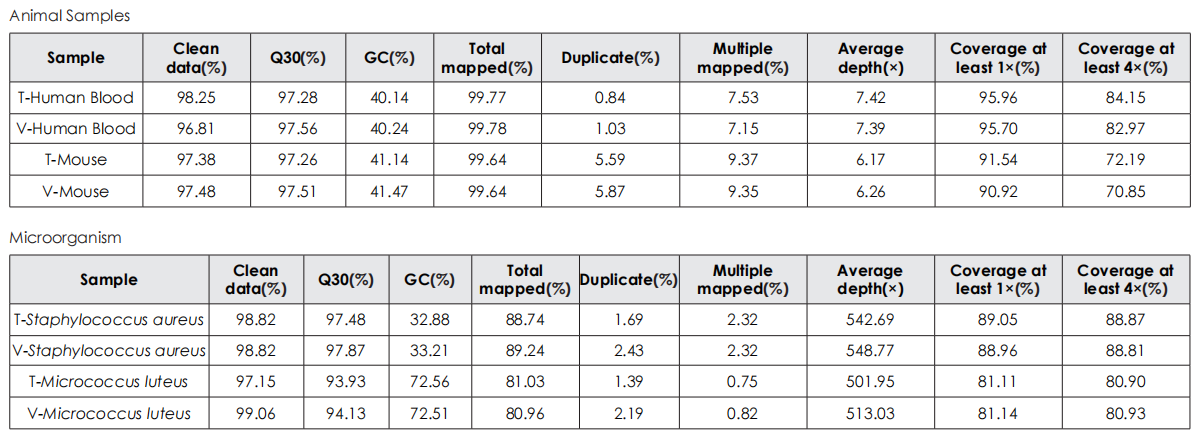
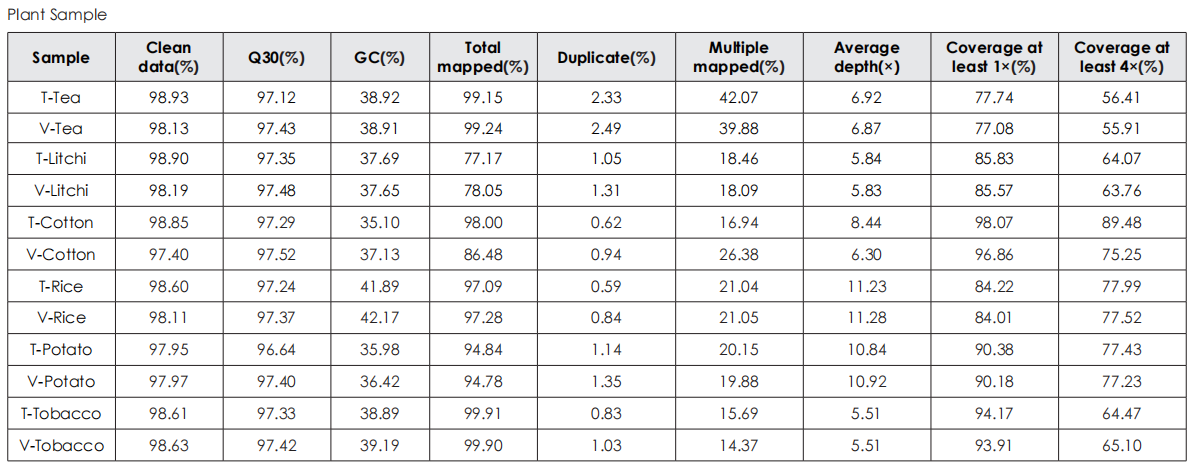
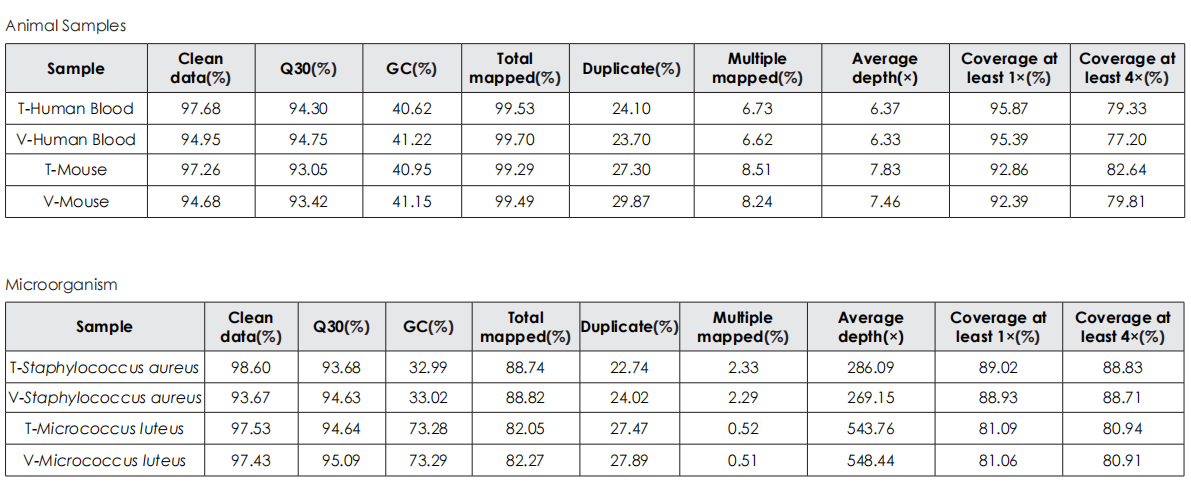
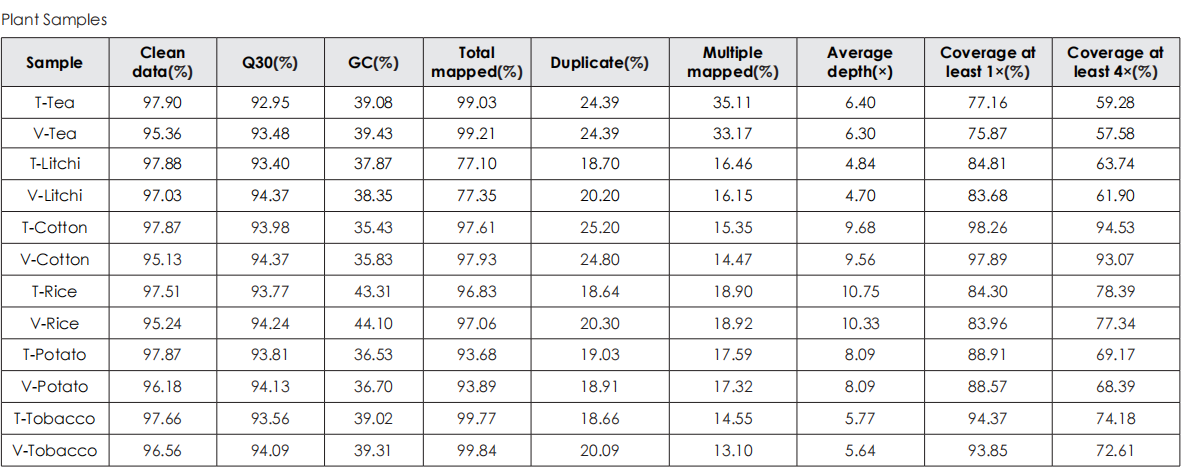
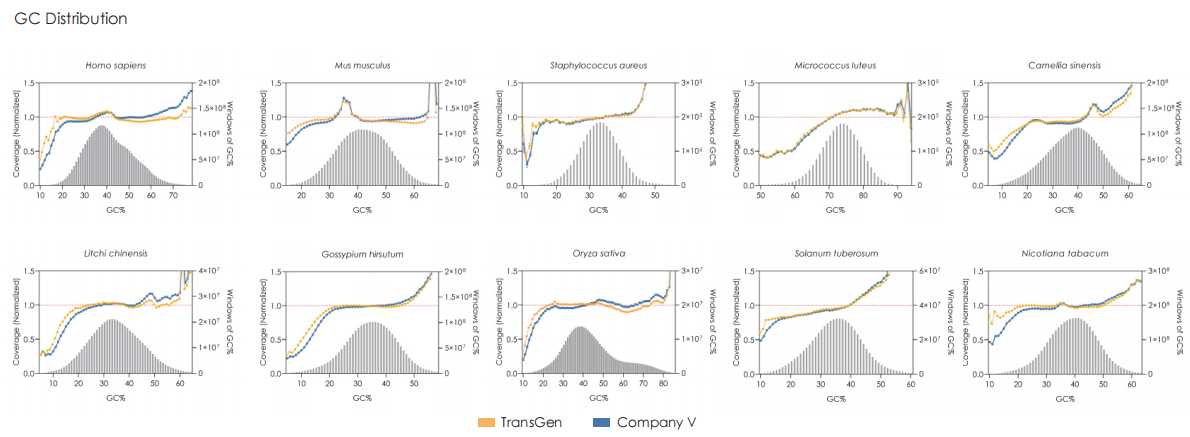
Sequencing Data Quality
Comparative evaluation of TransGen and Company V kits for library construction performance across diverse animal, plant, and microbial samples demonstrates comparable performance in key metrics, including sequencing quality, alignment rate, coverage uniformity, and GC distribution.
TransNGS® Fragmentase DNA Library Prep Kit for MGI®
This product enables rapid and efficient DNA library construction from 1 ng to 1 μg of input dsDNA, integrating DNA fragmentation, end repair, and A-tailing into a single-step reaction. The resulting product requires no intermediate purification and can be directly used for adapter ligation.
Schematic Diagram of Library Construction Principle
Comparative Analysis with Competing Products
Construct library using TransGen and Company V kits with varying input amounts (0.1 ng, 1 ng, 10 ng, and 100 ng) of HeLa cell genomic DNA. The TransGen kit demonstrates comparable or superior yields compared with Company V’s across all input ranges.
Comparative analysis across animal, plant and microbial samples shows equivalent sequencing quality to the competitor kits.